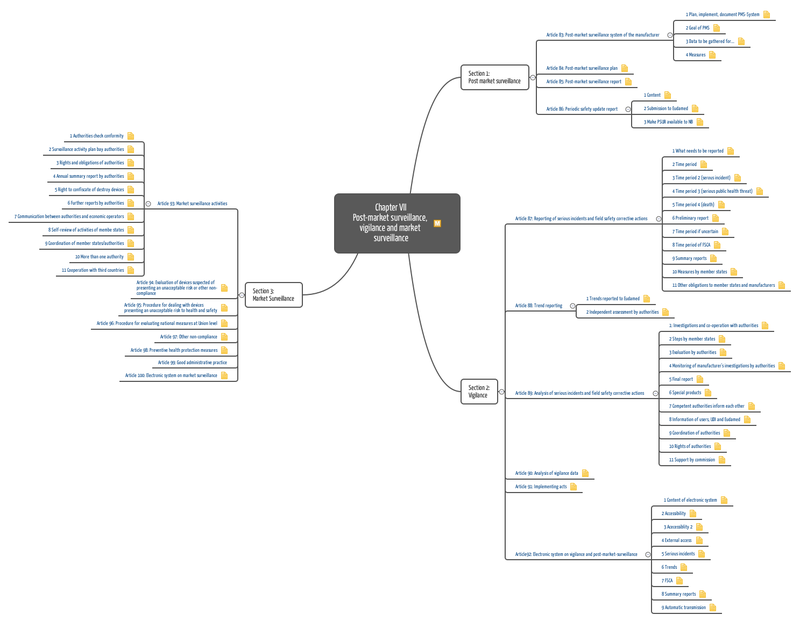
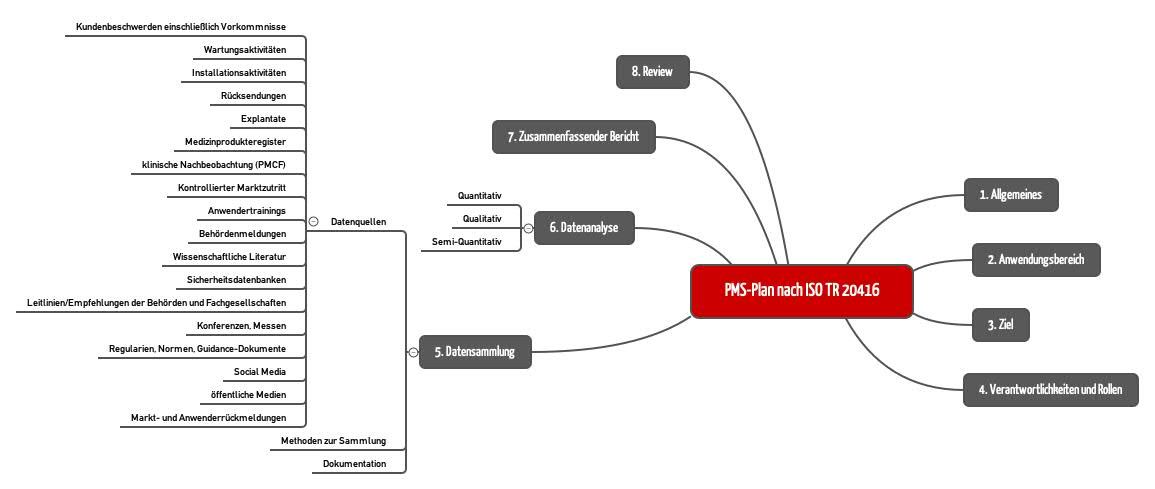
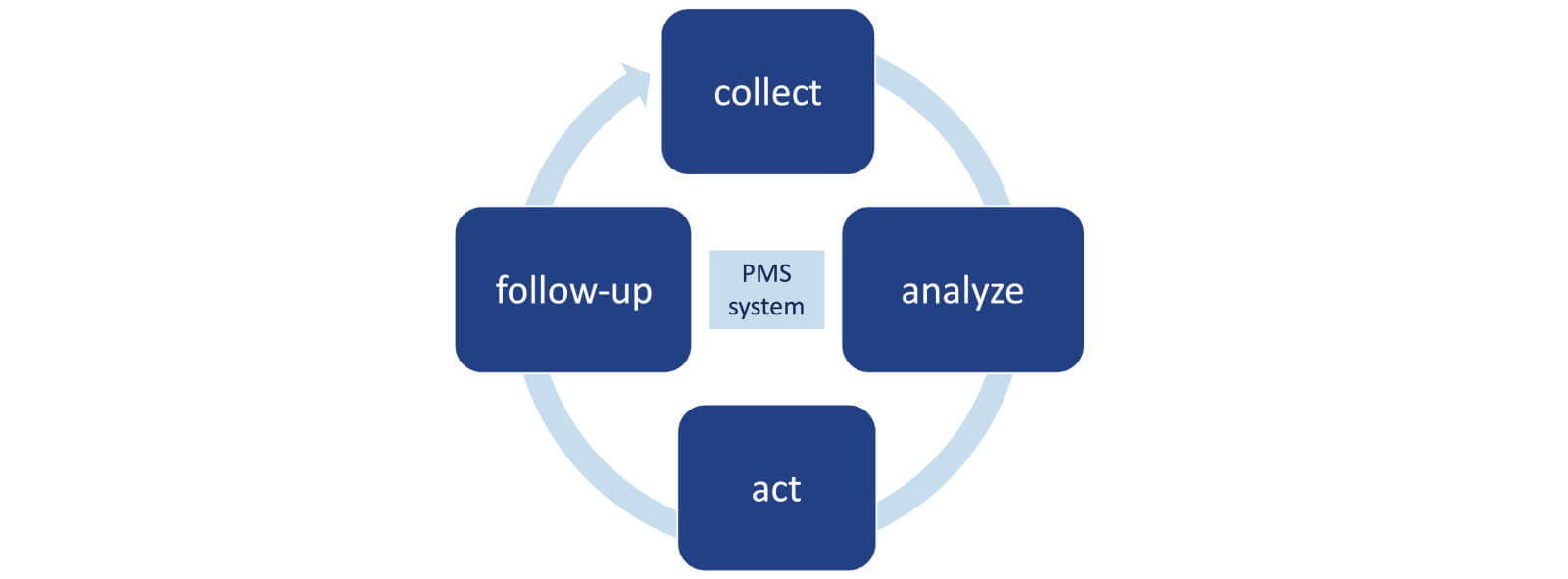
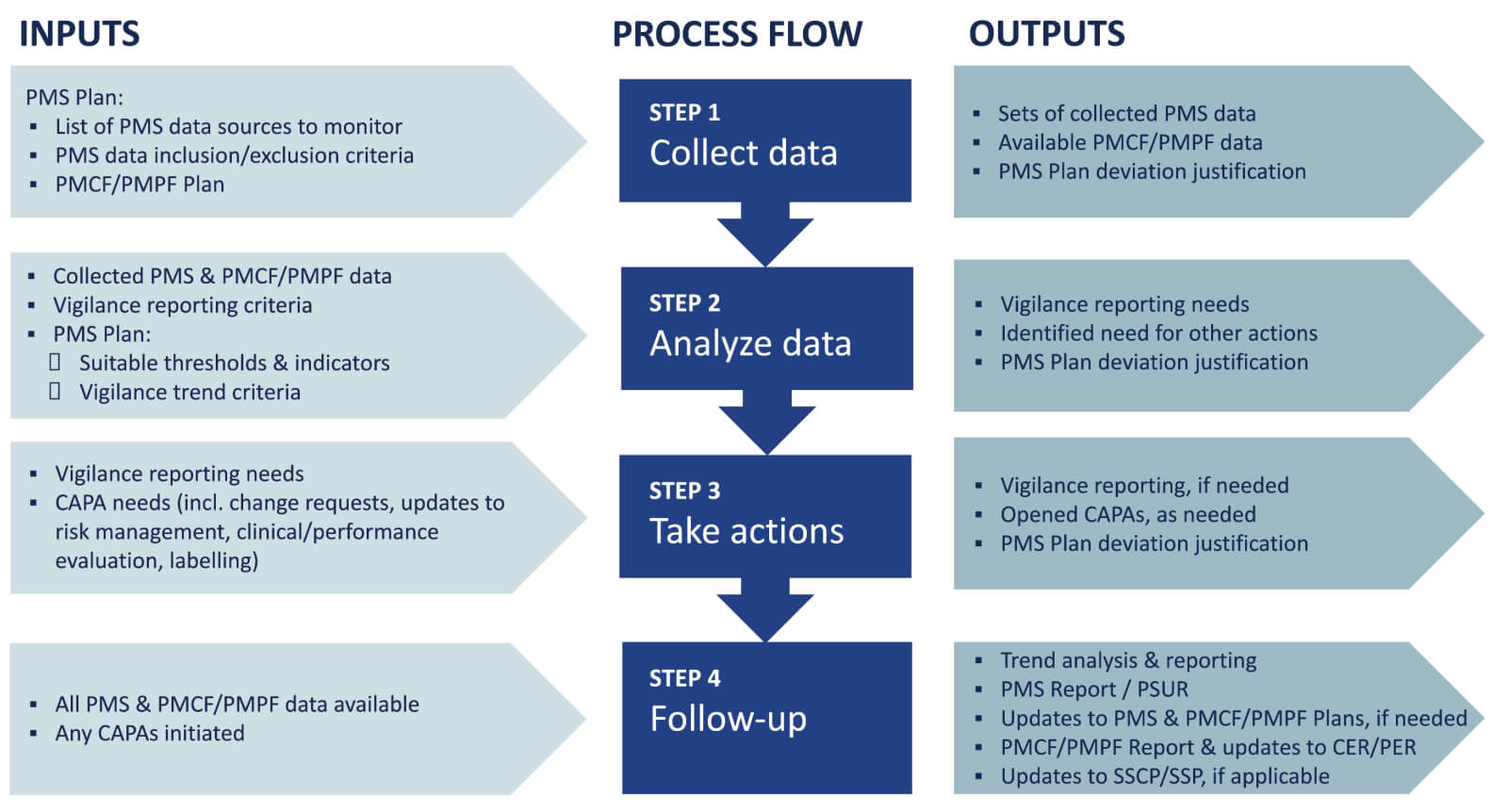
Guidance for post-market surveillance and market surveillance of medical devices, including in-vitro-diagnostics

EU postmarket surveillance plans for medical devices - Pane - 2019 - Pharmacoepidemiology and Drug Safety - Wiley Online Library

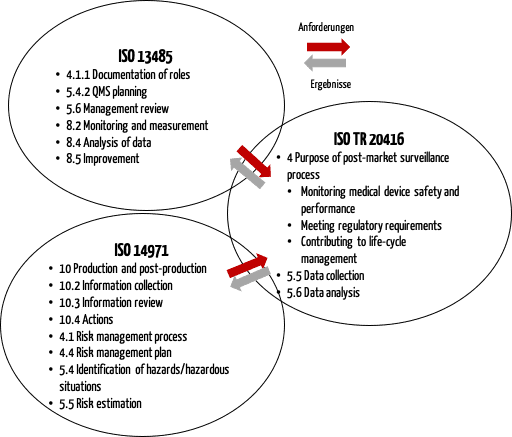
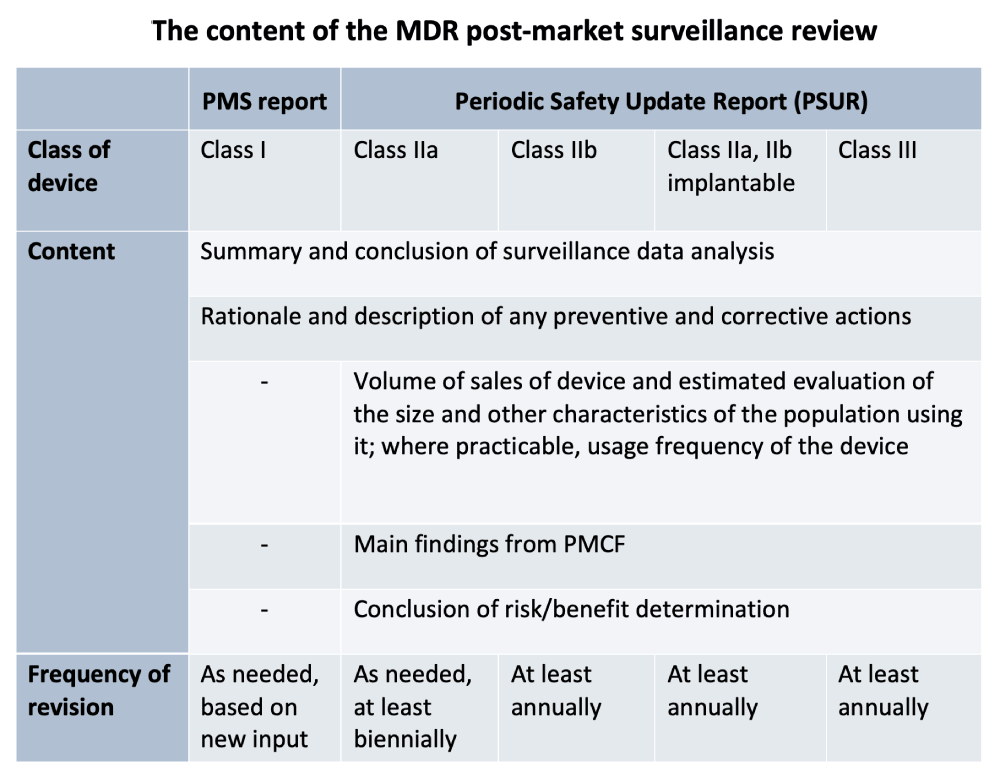
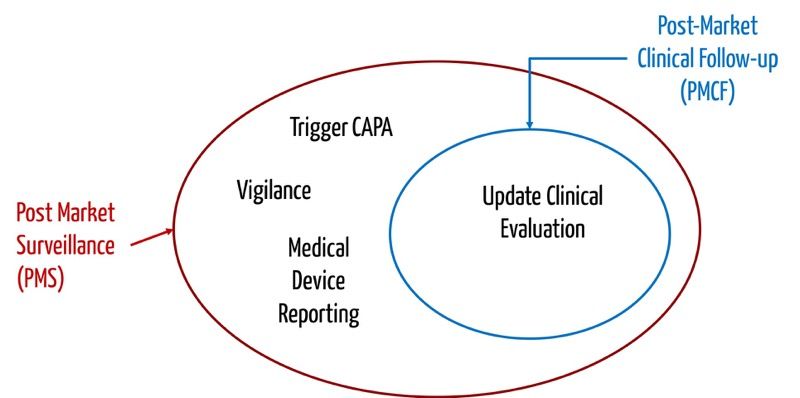






![Post-Market Surveillance Report [ISO 13485 templates] Post-Market Surveillance Report [ISO 13485 templates]](https://advisera.com/wp-content/uploads//sites/14/2021/08/18.2_Appendix_2_Post_Market_Surveillance_Report_Integrated_Preview_EN.png)
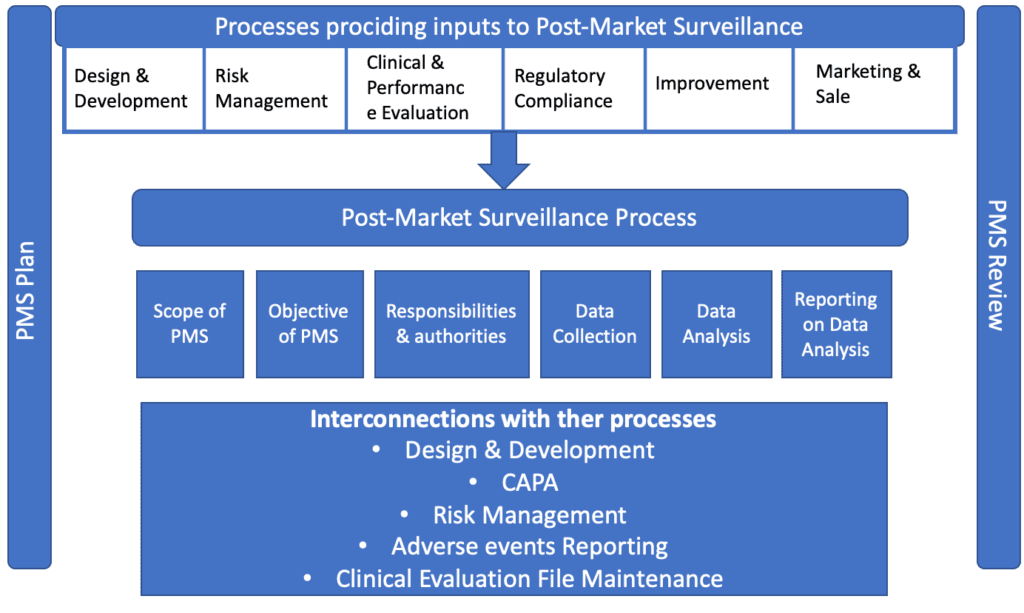





![Procedure for Post-Market Surveillance System [ISO 13485 templates] Procedure for Post-Market Surveillance System [ISO 13485 templates]](https://advisera.com/wp-content/uploads//sites/14/2021/08/18_Procedure_for_Post_Market_Surveillance_System_Integrated_Preview_EN.png)

