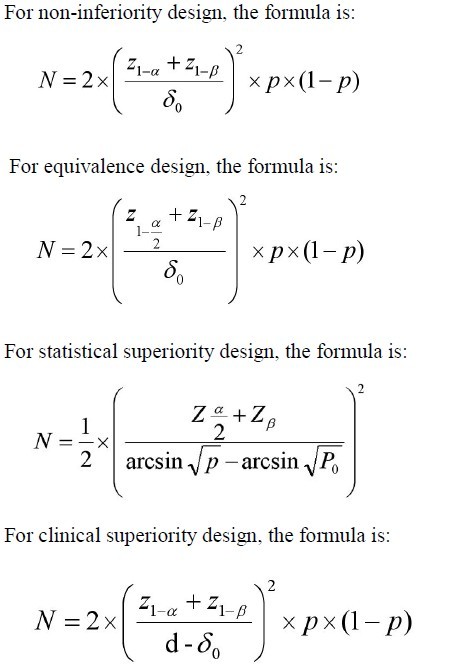
Differences in sample size for a non-inferiority trial when calculated... | Download Scientific Diagram
Sample Size Estimation for Non-Inferiority Trials: Frequentist Approach versus Decision Theory Approach | PLOS ONE

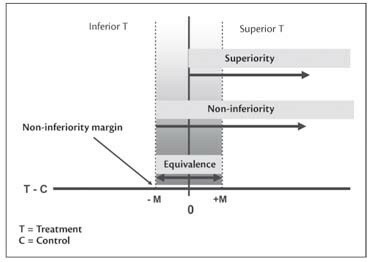
Figure 1 from Methodology of superiority vs. equivalence trials and non-inferiority trials. | Semantic Scholar
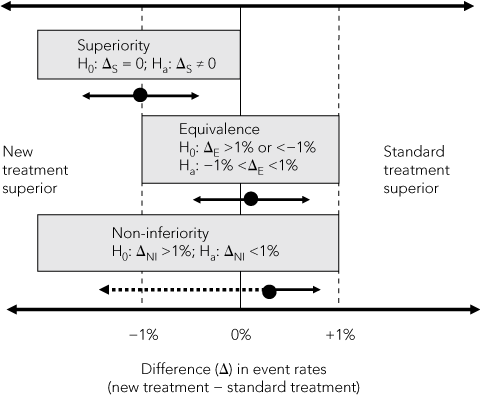
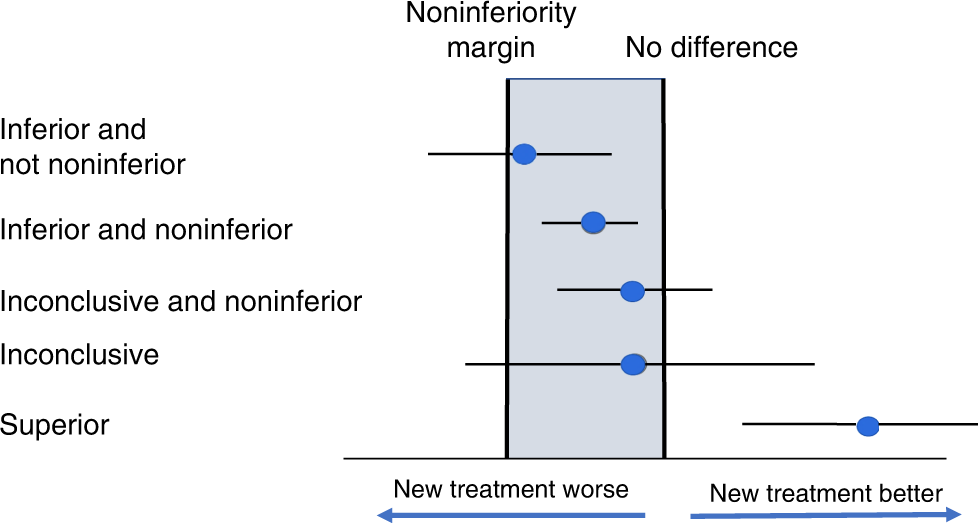
Non-inferiority trials: determining whether alternative treatments are good enough | The Medical Journal of Australia

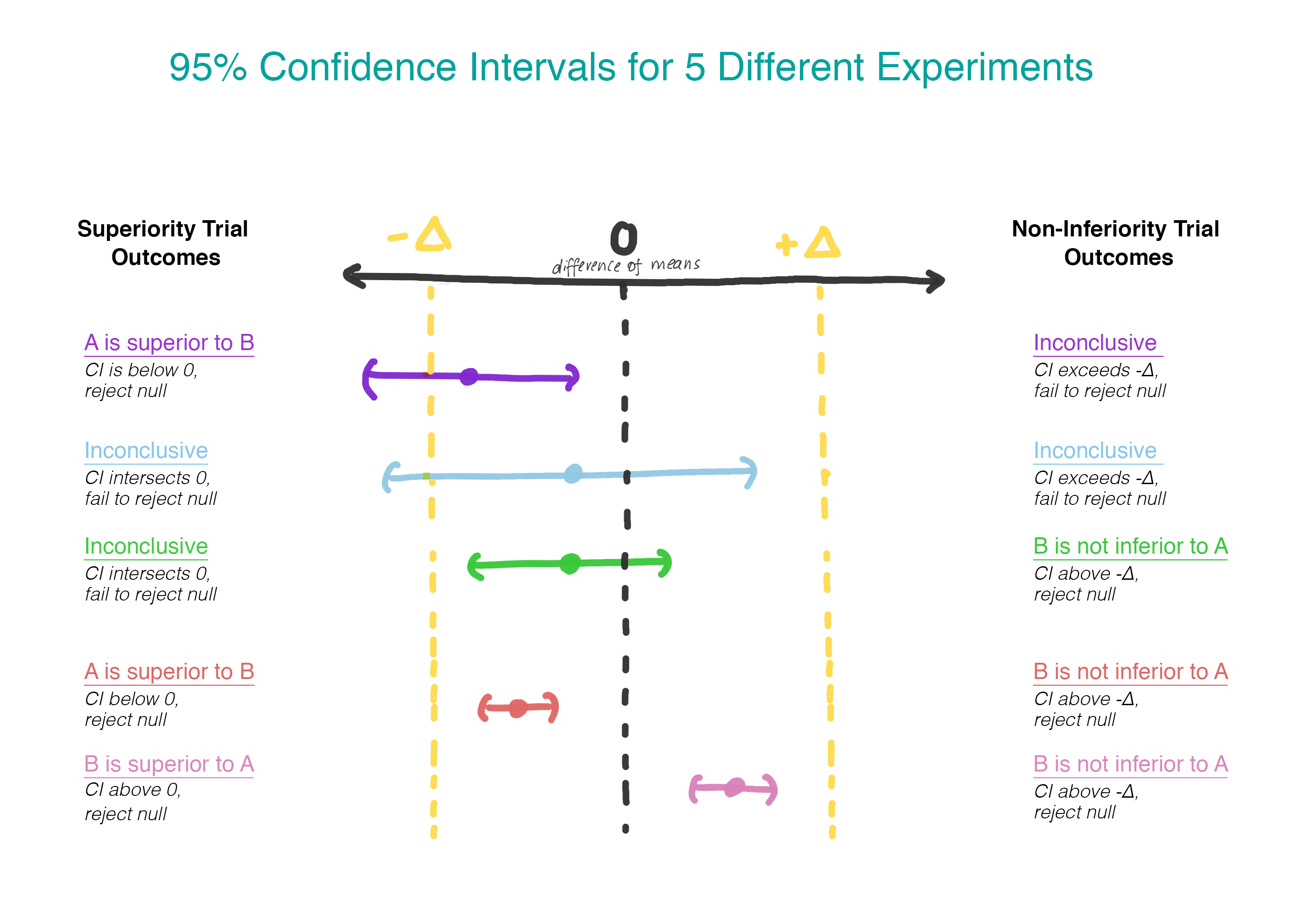
Figure. Non-inferiority margin and the relationship with 95% confidence... | Download Scientific Diagram