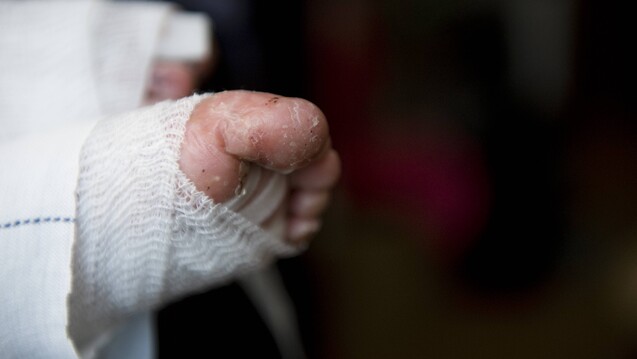
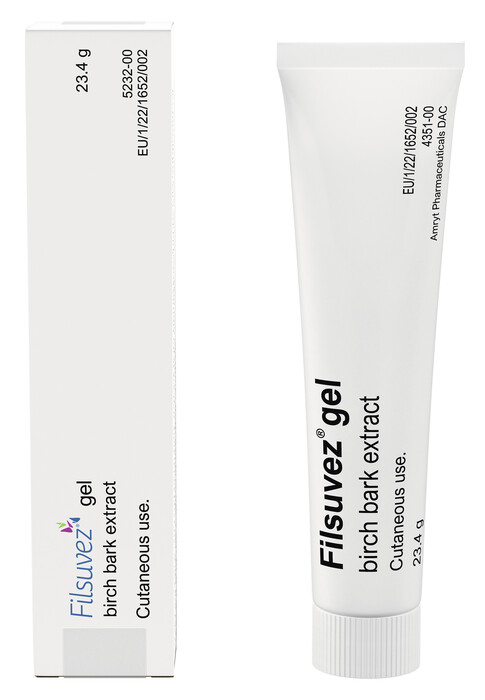
FDA Approves FILSUVEZ® (birch triterpenes) topical gel for the Treatment of EB - EB Research Partnership

Filsuvez, a topical gel, was FDA-approved on December 19, 2023, for epidermolysis bullosa ✓ - YouTube

FDA Approves FILSUVEZ® (birch triterpenes) topical gel for the Treatment of EB - EB Research Partnership