Pharmaceutics | Free Full-Text | Cereblon-Recruiting PROTACs: Will New Drugs Have to Face Old Challenges?

The rational design of CRBN-based PROTACs and chemical structures of... | Download Scientific Diagram

On the correlation of cereblon binding, fluorination and antiangiogenic properties of immunomodulatory drugs - ScienceDirect
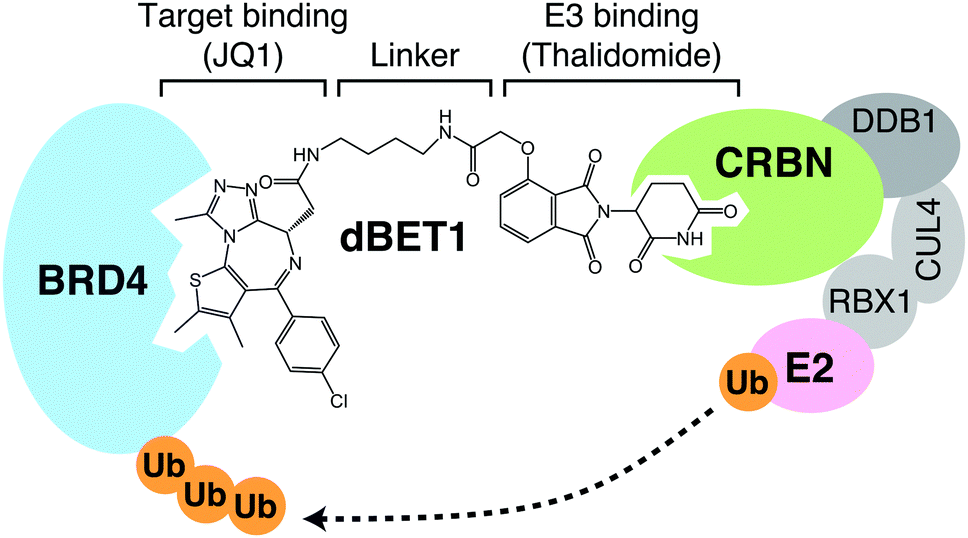
Discovery of CRBN as a target of thalidomide: a breakthrough for progress in the development of protein degraders - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00116K
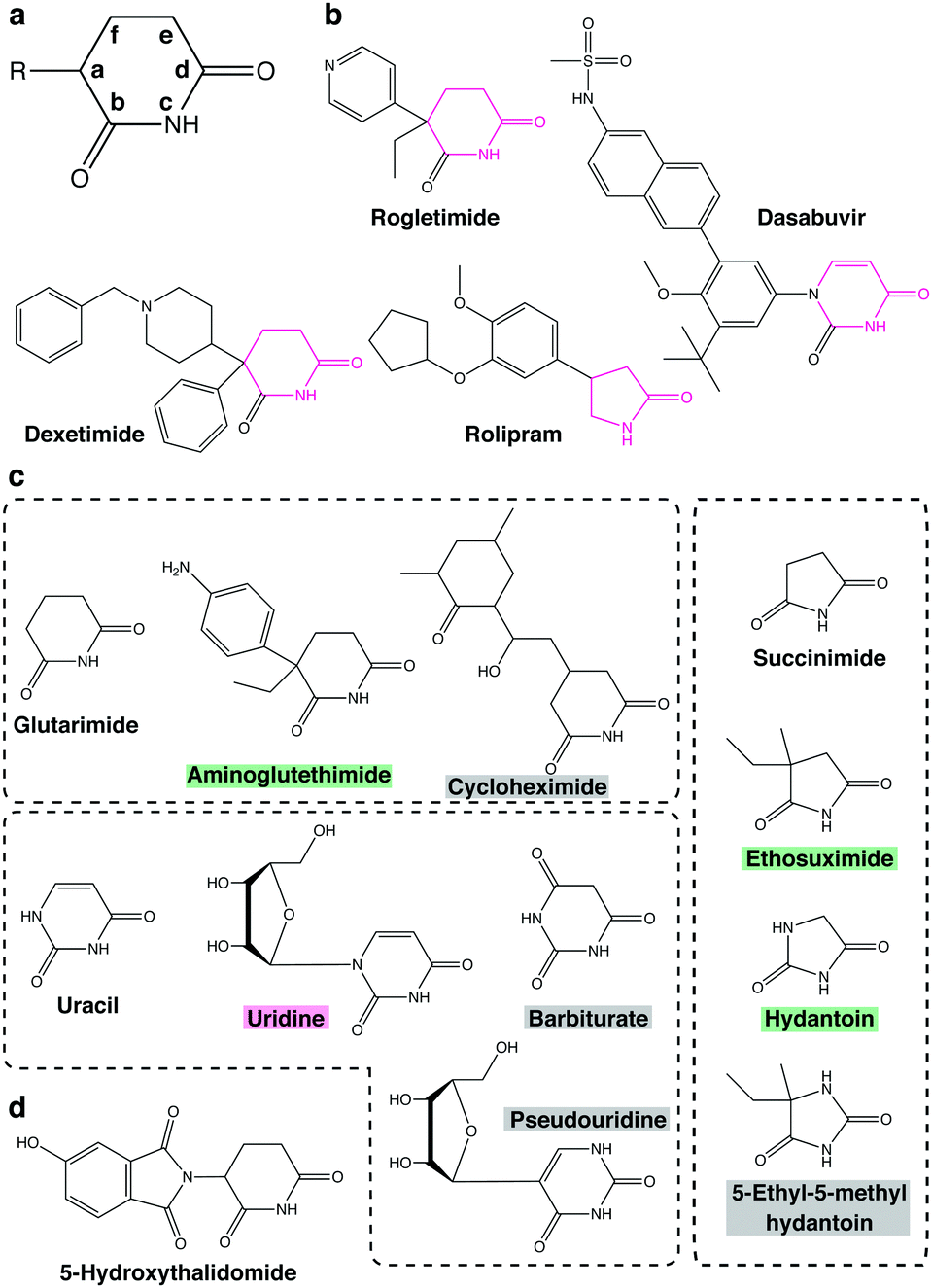
Discovery of CRBN as a target of thalidomide: a breakthrough for progress in the development of protein degraders - Chemical Society Reviews (RSC Publishing) DOI:10.1039/D2CS00116K
A Cell-Based Target Engagement Assay for the Identification of Cereblon E3 Ubiquitin Ligase Ligands and Their Applicati

The input for the PRosettaC protocol. A. The chemical structure of a... | Download Scientific Diagram
Counter Screen Identified the Putative Hits as Potent GSPT1 Degraders, Accounting for the Observed JAK2 Loss Co-dosing with JAK2

SYNthesis med chem: CRBN E3 Ligase Binder for your PROTAC molecule. | SYNthesis med chem posted on the topic | LinkedIn

Covalent Stapling of the Cereblon Sensor Loop Histidine Using Sulfur-Heterocycle Exchange | ACS Medicinal Chemistry Letters
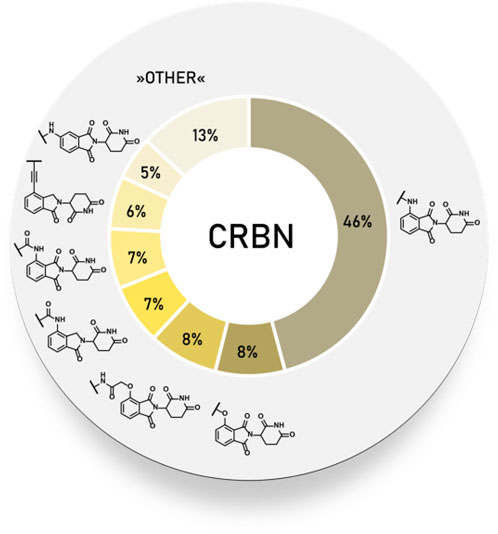
Frontiers | E3 Ligase Ligands in Successful PROTACs: An Overview of Syntheses and Linker Attachment Points

Enhancing Intracellular Accumulation and Target Engagement of PROTACs with Reversible Covalent Chemistry | bioRxiv
De-Novo Design of Cereblon (CRBN) Effectors Guided by Natural Hydrolysis Products of Thalidomide Derivatives
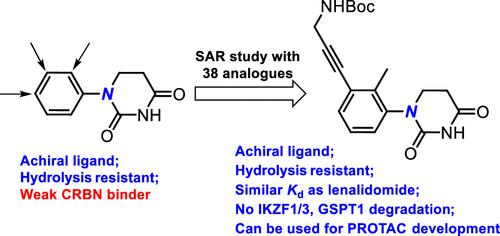
Development of Substituted Phenyl Dihydrouracil as the Novel Achiral Cereblon Ligands for Targeted Protein Degradation,Journal of Medicinal Chemistry - X-MOL