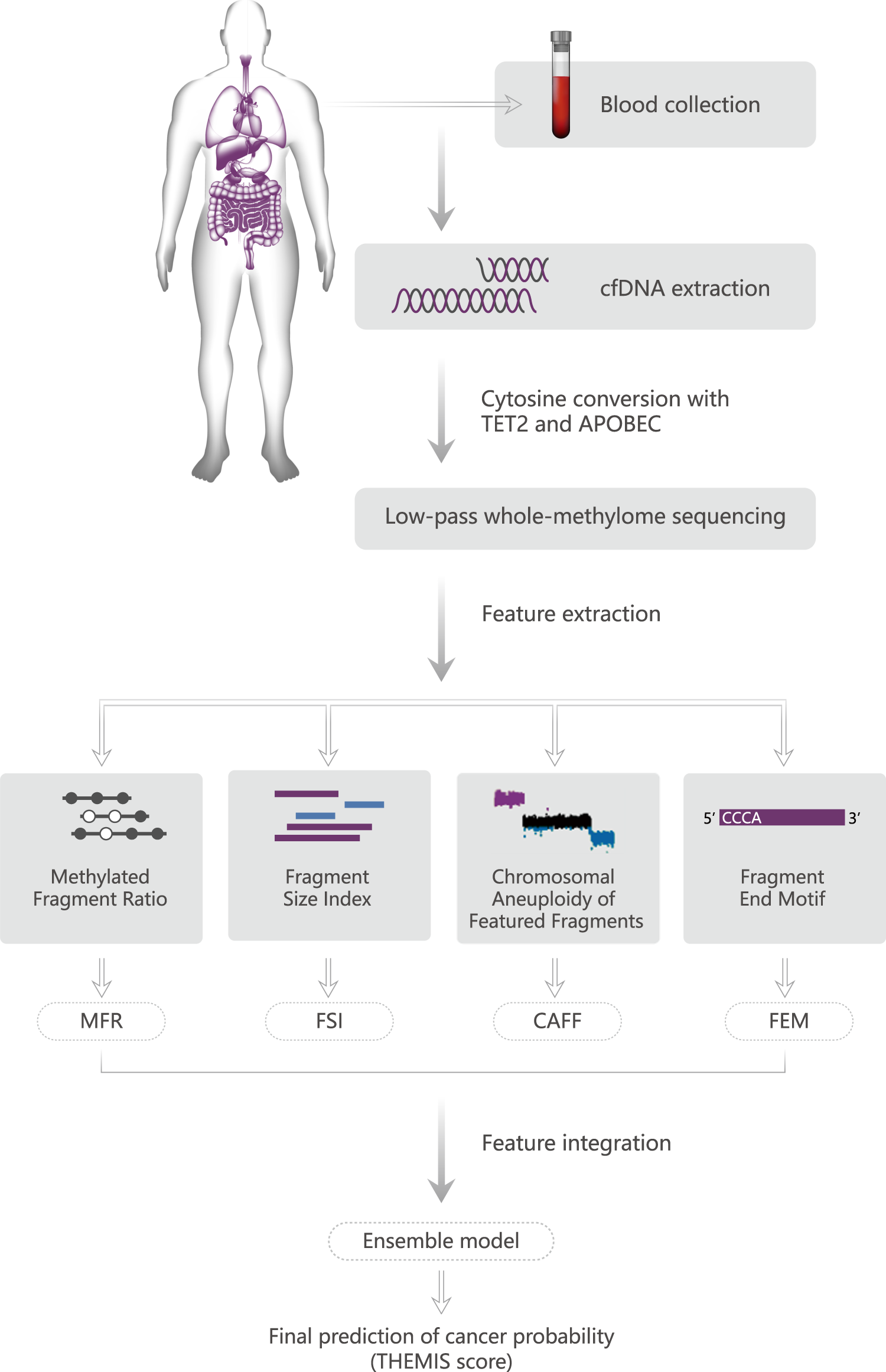
Multimodal analysis of cell-free DNA whole-methylome sequencing for cancer detection and localization | Nature Communications

Cell-free DNA methylation-defined prognostic subgroups in small-cell lung cancer identified by leukocyte methylation subtraction - ScienceDirect
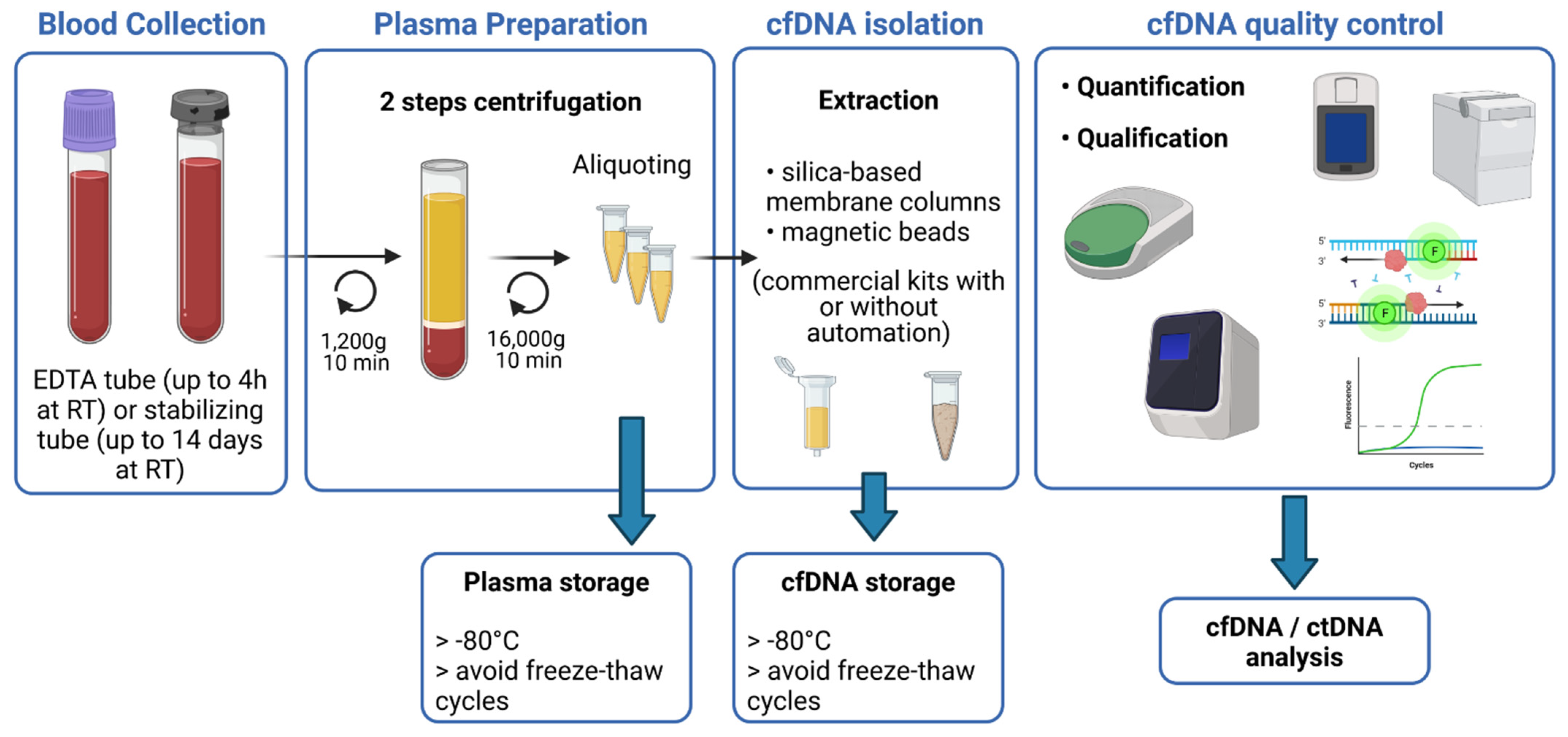
Pharmaceuticals | Free Full-Text | cfDNA Sequencing: Technological Approaches and Bioinformatic Issues

Comparison of methods for the isolation of cell-free DNA from cell culture supernatant - Abel Jacobus Bronkhorst, Vida Ungerer, Stefan Holdenrieder, 2020
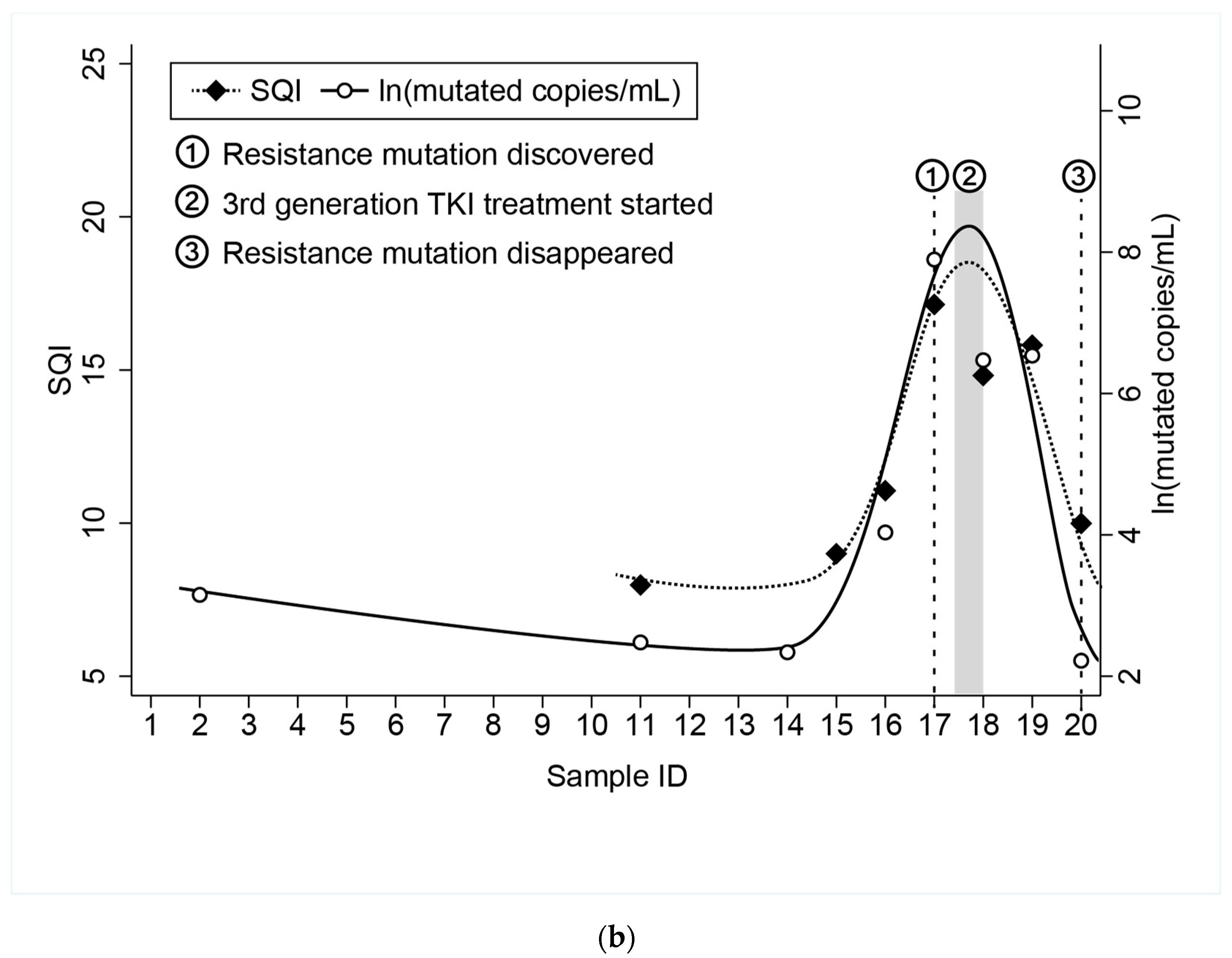
Diagnostics | Free Full-Text | Technical Evaluation of the COBAS EGFR Semiquantitative Index (SQI) for Plasma cfDNA Testing in NSCLC Patients with EGFR Exon 19 Deletions

Quantitative PCR–Based Method to Assess Cell-Free DNA Quality, Adjust Input Mass, and Improve Next-Generation Sequencing Assay Performance - ScienceDirect

A standardised methodology for the extraction and quantification of cell-free DNA in cerebrospinal fluid and application to evaluation of Alzheimer's disease and brain cancers - ScienceDirect

a) Electropherogram of cfDNA samples using the High Sensitivity D1000... | Download Scientific Diagram
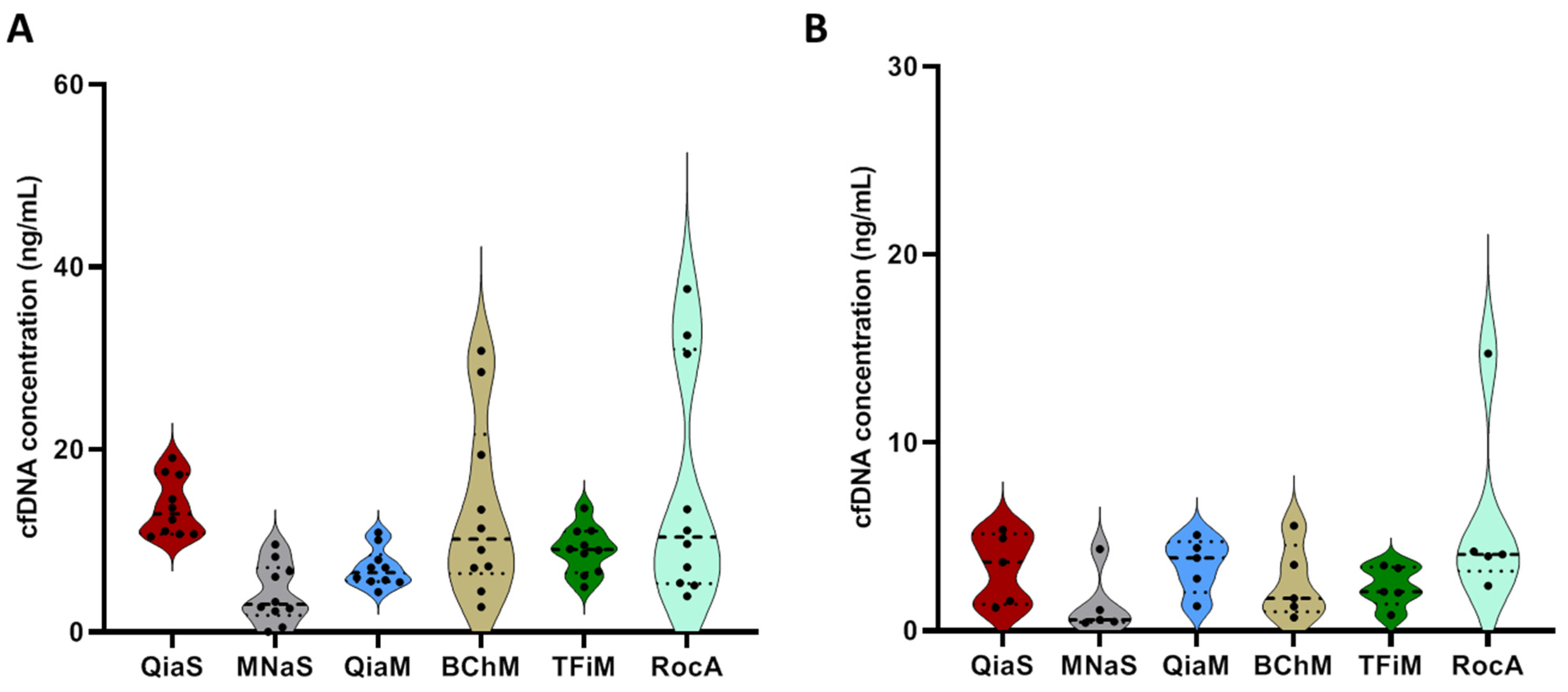
Diagnostics | Free Full-Text | Isolation and Quantification of Plasma Cell-Free DNA Using Different Manual and Automated Methods
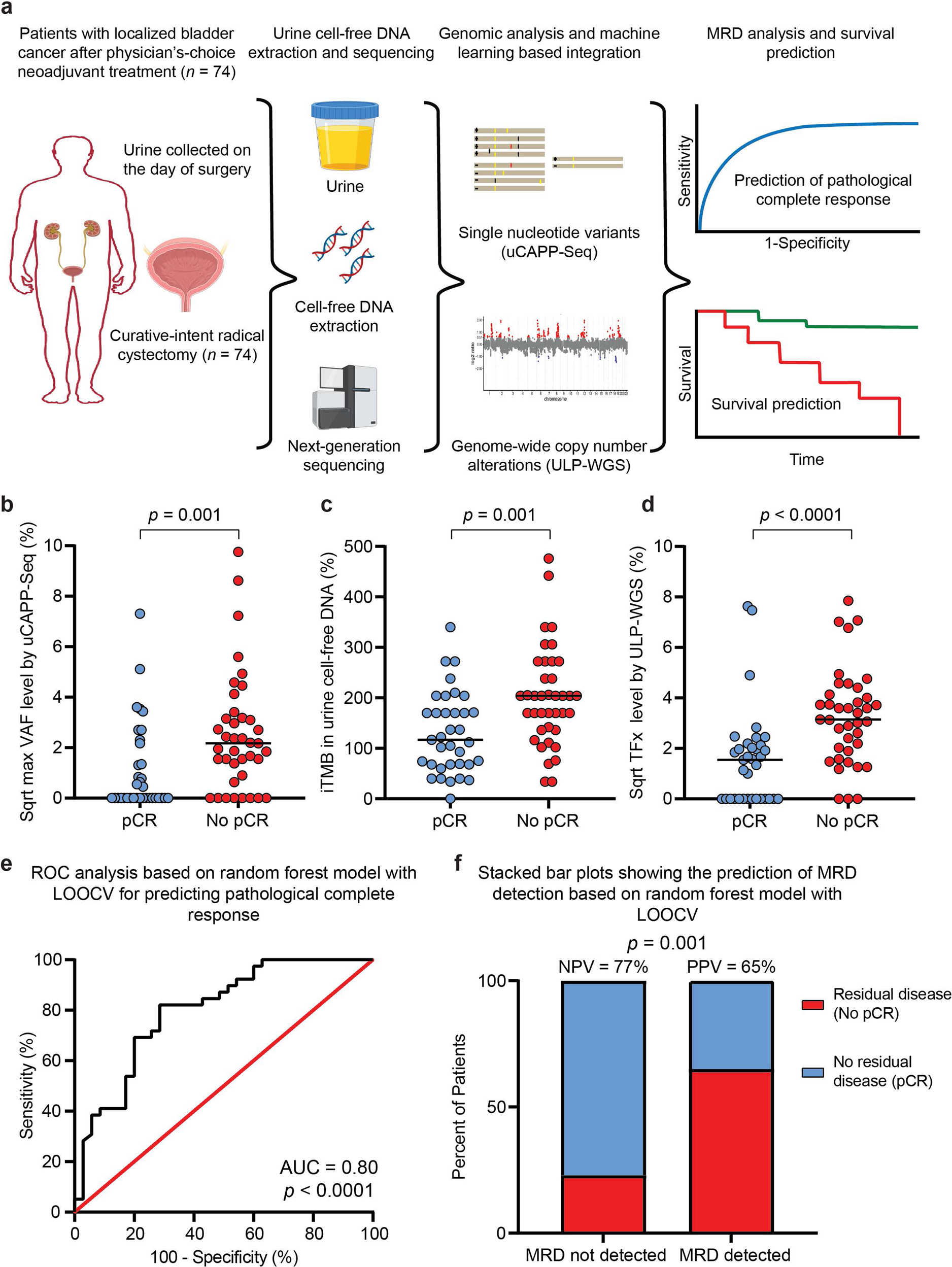
Urine cell-free DNA multi-omics to detect MRD and predict survival in bladder cancer patients | npj Precision Oncology
A Highly Verified Assay for KRAS Mutation Detection in Tissue and Plasma of Lung, Colorectal, and Pancreatic Cancer - Document - Gale OneFile: Health and Medicine
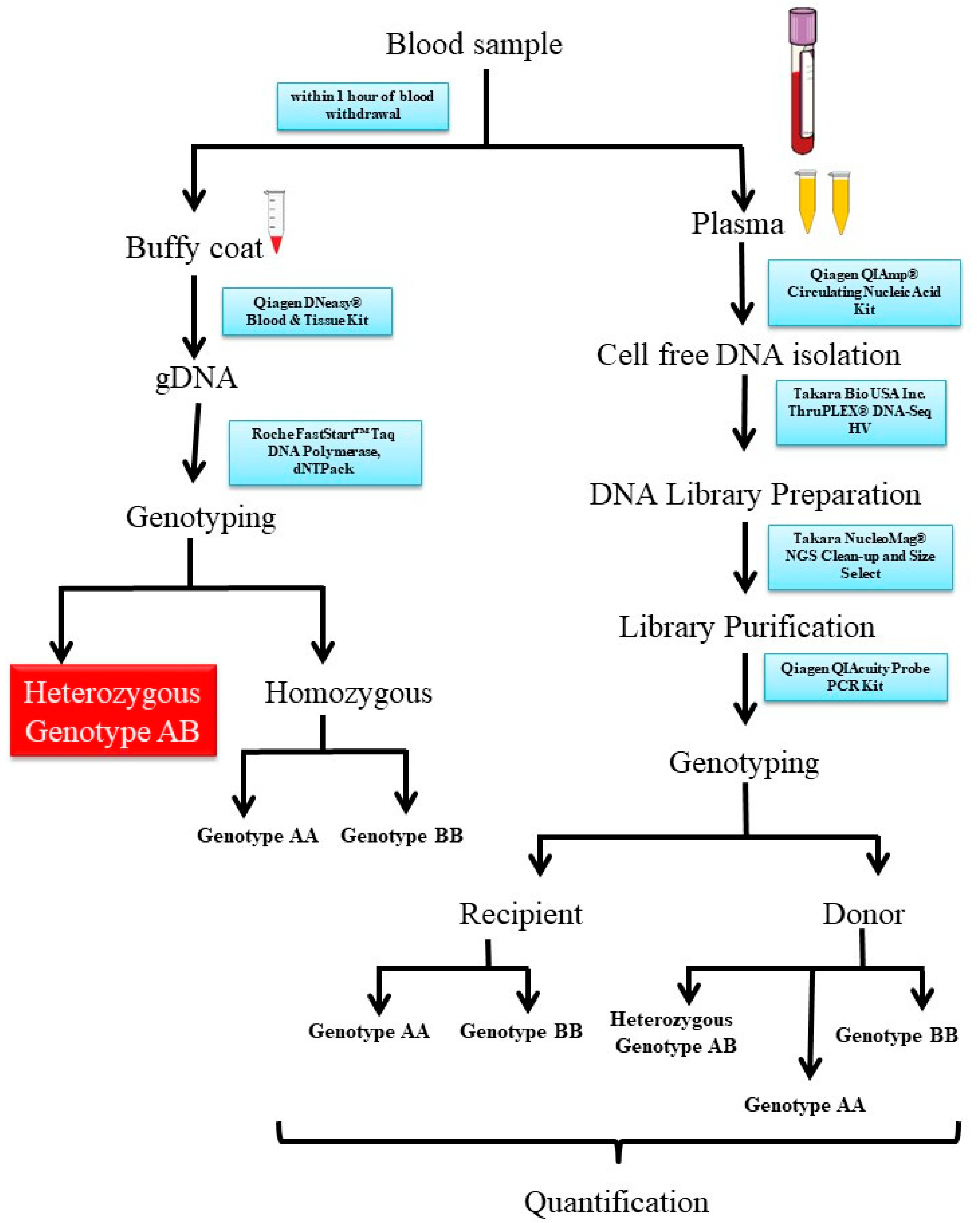
Diagnostics | Free Full-Text | Donor-Derived Cell-Free DNA as a Non-Invasive Biomarker for Graft Rejection in Kidney Transplant Recipients: A Prospective Study among the Indian Population
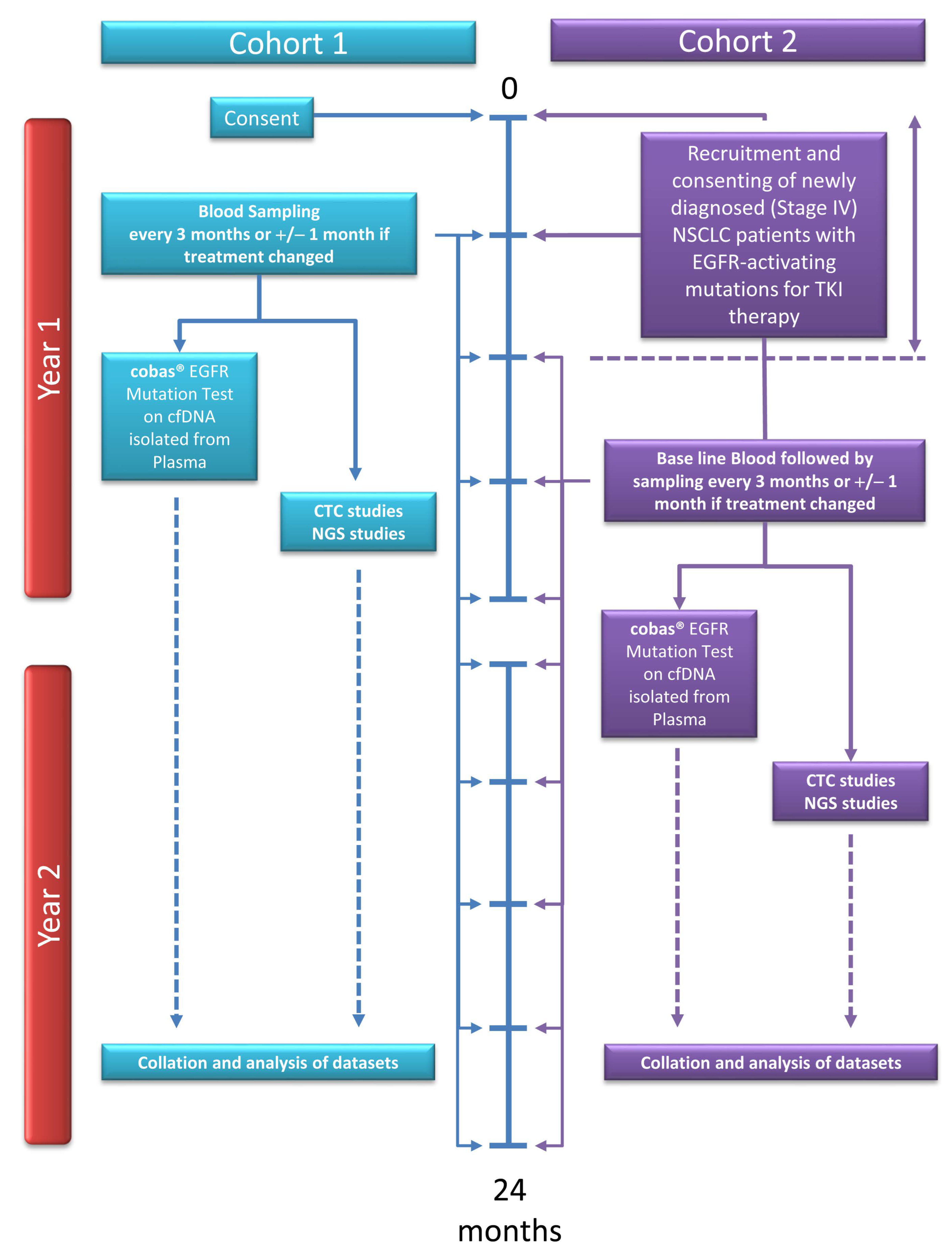
Diagnostics | Free Full-Text | Liquid Biopsy: A Multi-Parametric Analysis of Mutation Status, Circulating Tumor Cells and Inflammatory Markers in EGFR-Mutated NSCLC

Digital PCR-based evaluation of nucleic acid extraction kit performance for the co-purification of cell-free DNA and RNA | Human Genomics | Full Text