If the binding energy per nucleon in `L i^7` and `He^4` nuclei are respectively `5.60 MeV` and ` - YouTube

A density functional theory study of high-performance pre-lithiated MS2 (M = Mo, W, V) Monolayers as the Anode Material of Lithium Ion Batteries | Scientific Reports
Binding energy of each Li ion on C 60 and C 60 H 18 as a function of... | Download Scientific Diagram

Mechanical Bond Enhanced Lithium Halide Ion‐Pair Binding by Halogen Bonding Heteroditopic Rotaxanes** - Munasinghe - 2022 - Chemistry – A European Journal - Wiley Online Library

Lithium and Sodium Ion Binding Mechanisms and Diffusion Rates in Lignin-Based Hard Carbon Models | ACS Omega

Proficient electron injection lithium complexes designed by molecular energy calculation for high performance OLEDs - ScienceDirect

Figure 4 from A Biodegradable Polydopamine-Derived Electrode Material for High-Capacity and Long-Life Lithium-Ion and Sodium-Ion Batteries. | Semantic Scholar

Cost-Effective Water-Soluble Poly(vinyl alcohol) as a Functional Binder for High-Sulfur-Loading Cathodes in Lithium–Sulfur Batteries | ACS Omega

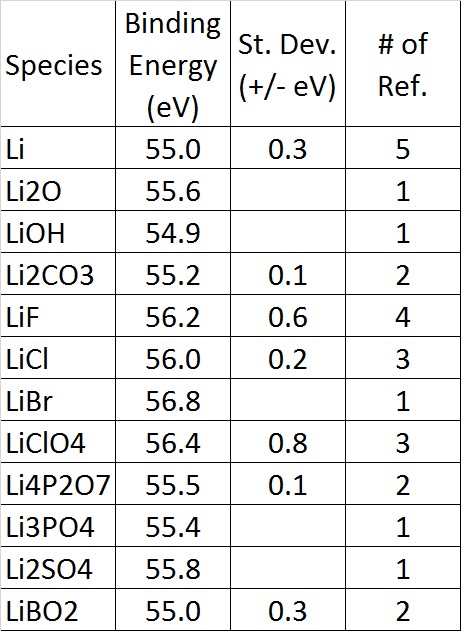
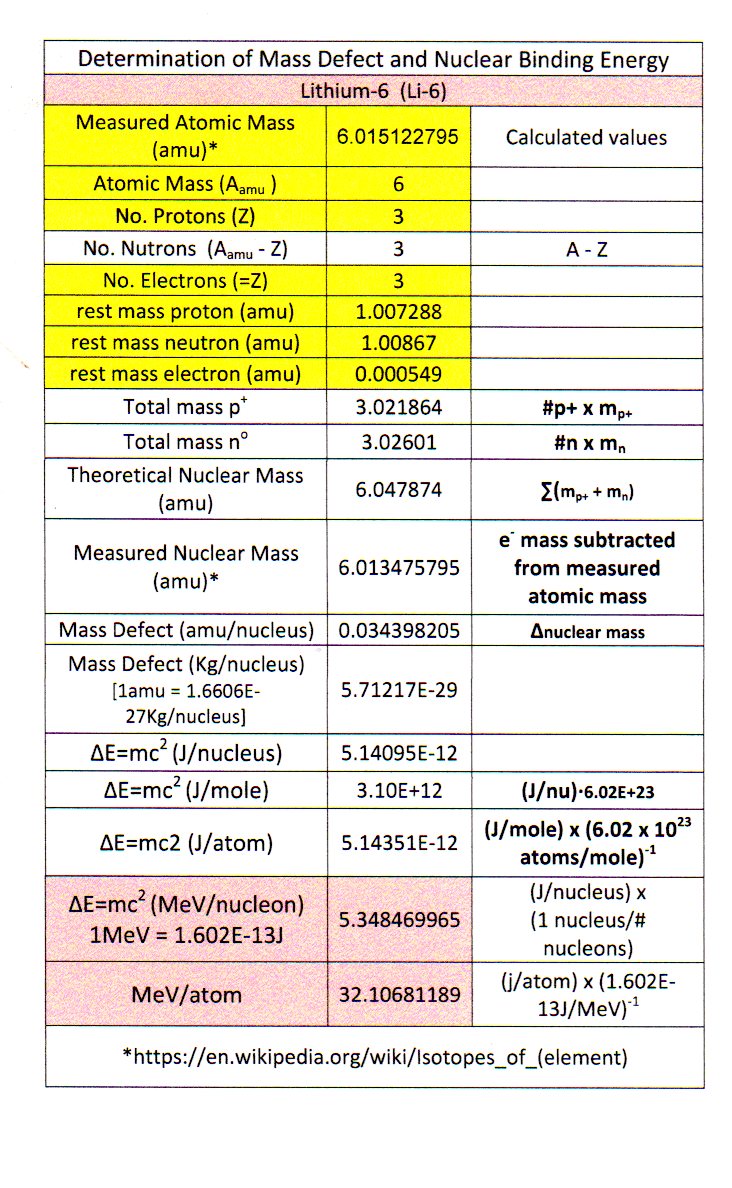
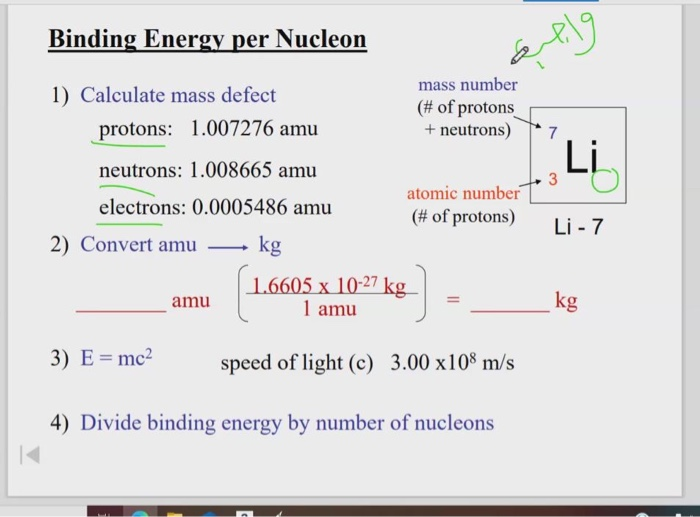
Find the binding energy of the nucleus of lithium isotope _{3}{Li}^{7} and hence the binding energy per nucleon in it.left({ M }_{ _{ 3 }{ Li }^{ 7 } }=7.014353amu, { M }_{ _{

A density functional theory study of high-performance pre-lithiated MS2 (M = Mo, W, V) Monolayers as the Anode Material of Lithium Ion Batteries | Scientific Reports
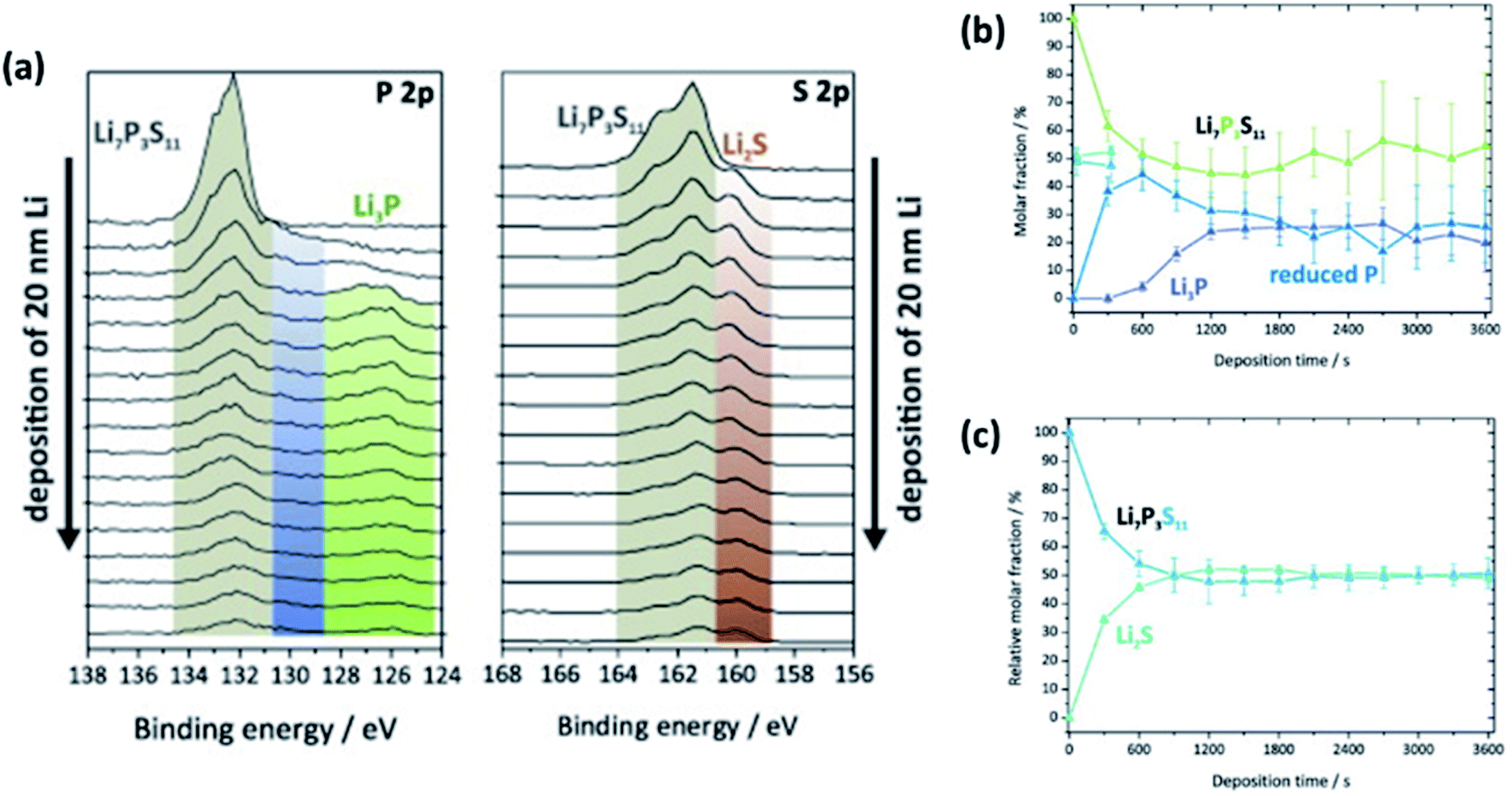
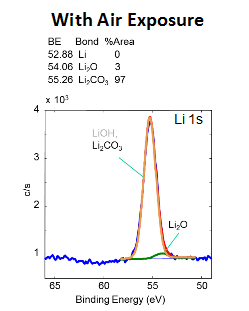
Advances in studying interfacial reactions in rechargeable batteries by photoelectron spectroscopy - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/D2TA03242B
Ionisation energy of Li atom in ground state is 5.4 eV.Binding energy of an electron in Li+ Ion in ground state is 75.6 eV.Energy required to remove all three electrons of Li

Binding properties of some alkali metal vapors: specifically lithium-7 (7Li), soduim-23 (23Na), and potassium-39 (39K)