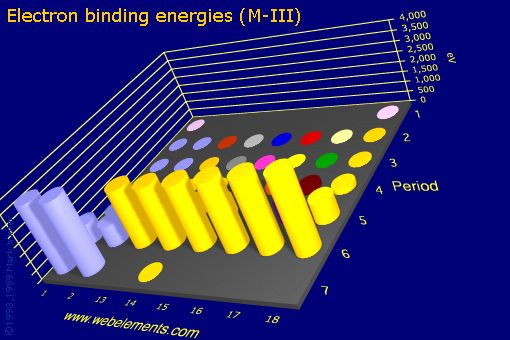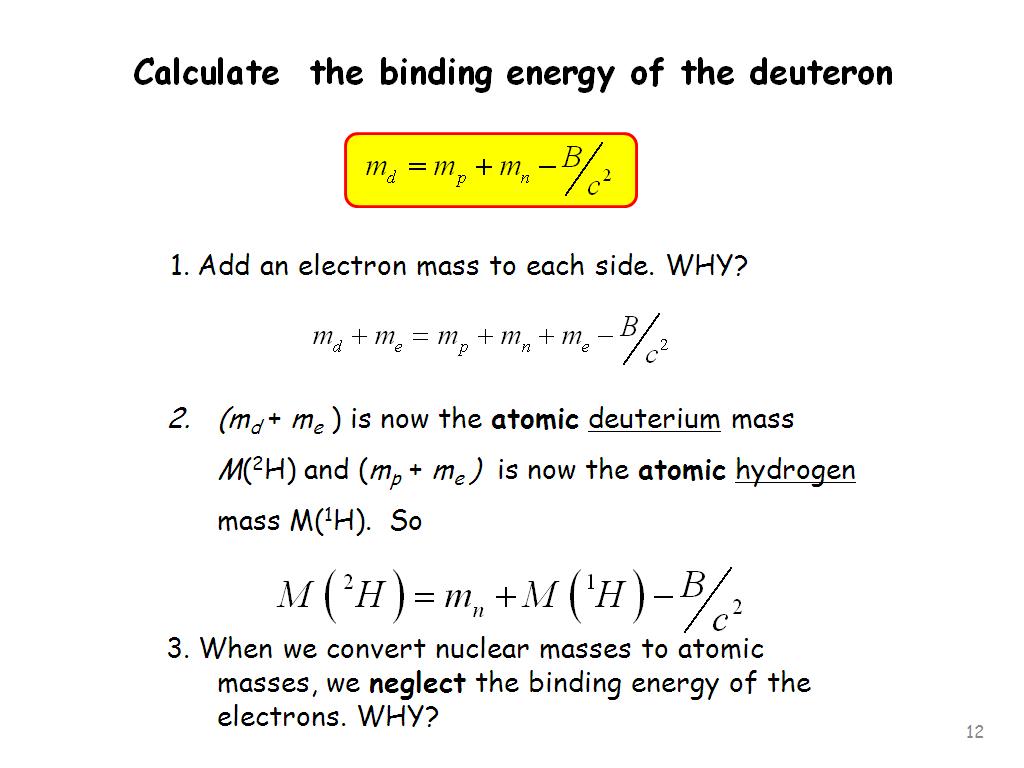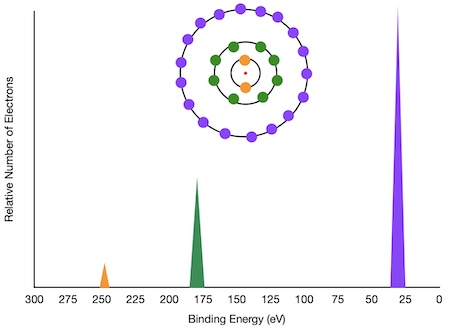
Relating the Relationship the Photoelectron Spectrum to the Interactions of the Nucleus and the Electrons. | Chemistry | Study.com
97 binding energy of electrons in ground state of Hatomis 13.6eV ,energies required to eject out an electrons from three lowest states of He + atom willl be 1)13.6,10.2,3.4 2)13.6,3.4,1.5 3)13.6,27.2,40.8 4)54.5,13.6,6 explain??
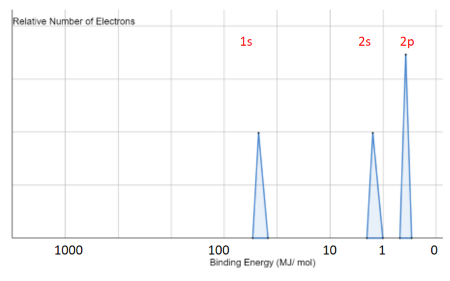
Relating the Relationship between the Photoelectron Spectrum and the Electron Configuration of the Species | Chemistry | Study.com

In a hydrogen atom, the binding energy of the electron in the ground state is `E_(1)` then - YouTube

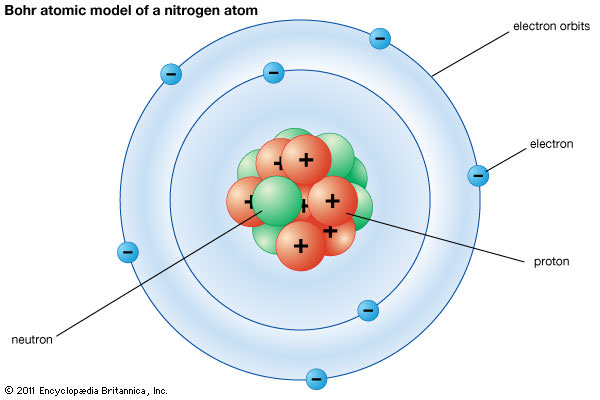
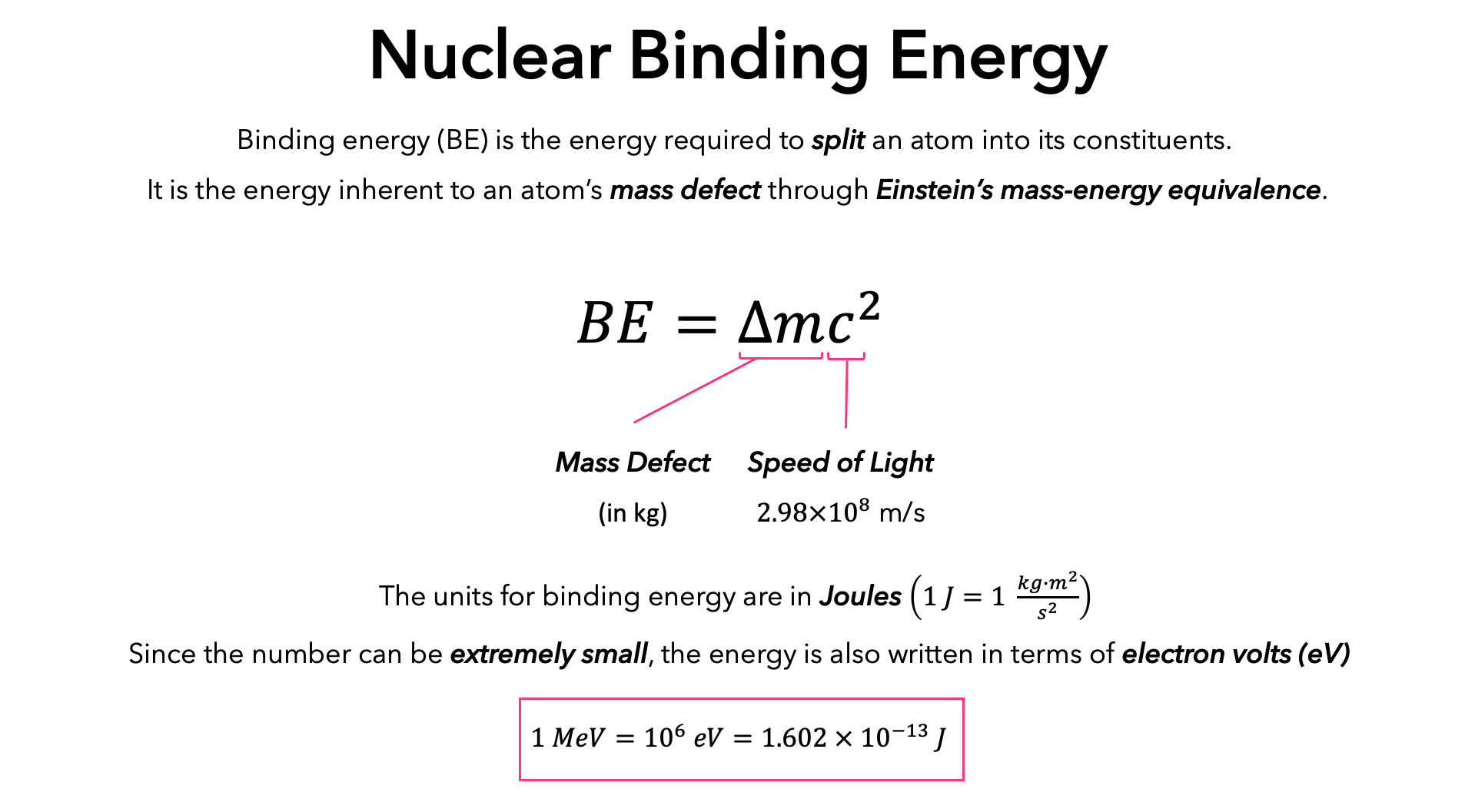
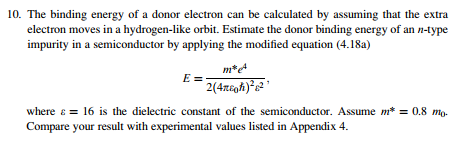



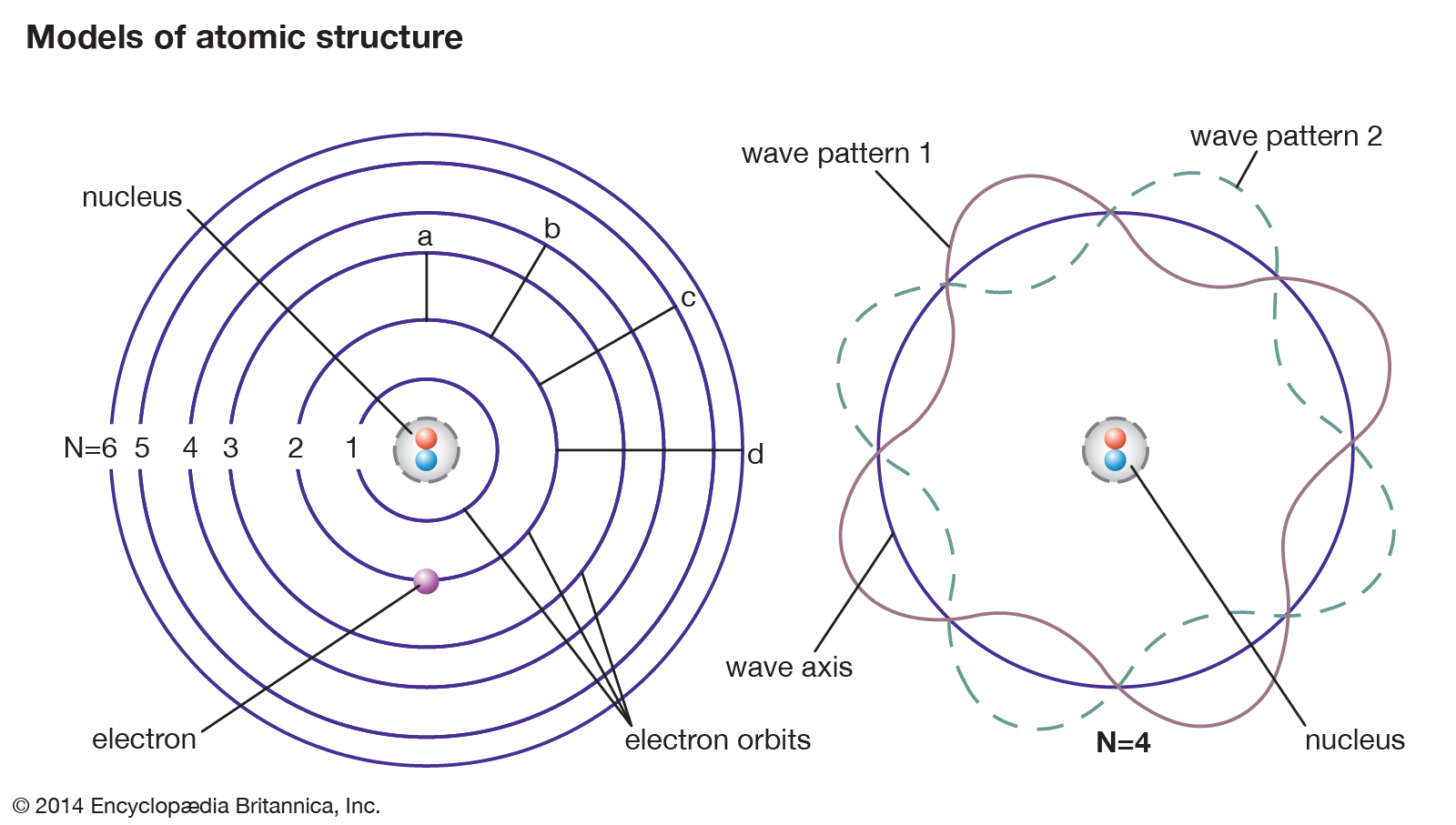
![Electron binding energies (in keV) for the rare gas atoms [22]. | Download Table Electron binding energies (in keV) for the rare gas atoms [22]. | Download Table](https://www.researchgate.net/publication/236670280/figure/tbl1/AS:651530181021698@1532348308530/Electron-binding-energies-in-keV-for-the-rare-gas-atoms-22.png)